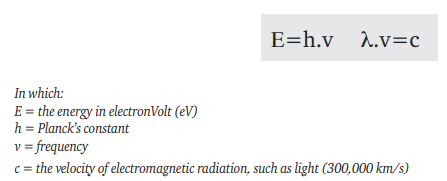What are X-rays?
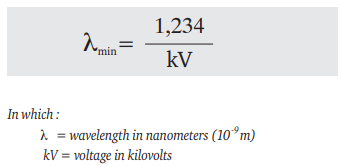
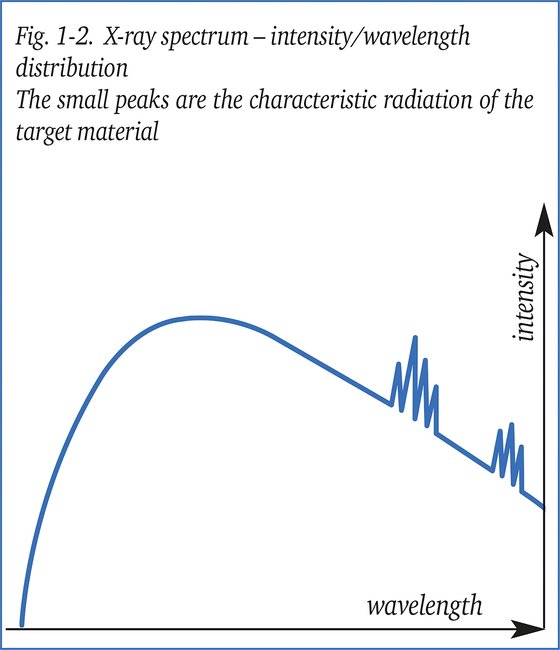
The radiation which is emitted by an X-ray tube is heterogeneous, that is, it contains X-rays of a number of wavelengths, in the form of a continuous spectrum with some superimposed spectrum lines. The shortest wavelength of the spectrum is given by the Duane-Hunt formula:
The average shape of the X-ray spectrum is generally the same however not truely identical for different X-ray sets; it depends chiefly on the energy range of the electrons striking the X-ray tube target and, therefore, on the voltage waveform of the high-voltage generator. A constant potential (CP) X-ray set will not have the same spectrum as a self-rectified set operating at the same nominal kV and current. The spectrum shape also depends on the inherent filtration in the X-ray tube window (glass, aluminium, steel or beryllium). The energy imparted to an electron having a charge e, accelerated by an electrical potential V is (eV) so the energy of the electrons can be quoted in eV, keV, MeV. These same units are used to denote the energy of an X-ray spectrum line. The energy of a single wavelength is:
The heterogeneous X-rays emitted by an X-ray tube do not however have a single wavelength, but a spectrum, so it would be misleading to describe the X-rays as (say) 120 keV X-rays. By convention therefore, the ‘e’- in keV- is omitted and the X-rays described as 120 kV, which is the peak value of the spectrum.