NDT medical solutions for product manufacturing and product quality inspection
The medical technology industry faces multilateral inspection challenges when manufacturing products that have a direct impact on the quality of many people’s lives. Product safety and reliability are crucial. High and stringent quality and safety standards have to be met. At the same time, the technology applied in many devices is ever more complex, implants are becoming smaller and high-tech electronic components are increasingly deployed. Product development cycles and production processes on the other hand continue to speed up.
We offer world leading CT-image quality to facilitate your full-cycle quality control

Detect structural defects in electronic or mechanical parts, orthopedic implants and dental applications, or need high precision metrology for the inspection of part dimensions

From pacemakers and other battery powered implants like nerve stimulators or cochlear implants, to orthophedic implants, dental replacements & brackets, catheters, surgical staples to hearing aids and insulin pumps

Quality control management all over the world still largely relies on manual workflows of visual inspection, with paper-based documentation that is in urgent need of better regulation and traceability

Safeguard 100% quality control while facilitating faster product design, more productivity and lower costs, and meet the challenges of OEMs and suppliers.
From pacemakers to orthophedic implants, dental replacements and brackets, catheters, surgical staples to hearing aids and insulin pumps
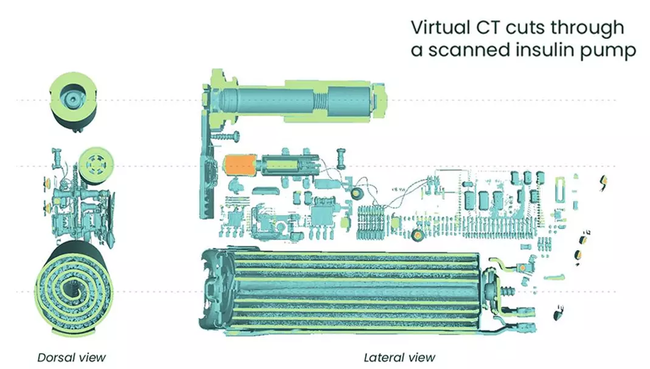

Technologies made for NDT medical inspections

Our powerful industrial computer tomography (CT) system, designed for 3D metrology and analysis, provides industry-leading magnification at 300 kV.
Why choose Waygate Technologies
Advanced NDT Solutions for Medical Device Reliability and Quality
Waygate Technologies offers cutting-edge non-destructive testing (NDT) solutions specifically designed to enhance the reliability and quality of medical products. Our advanced X-ray and Computed Tomography (CT) inspection methods are crucial for identifying structural and soldering defects in critical electronic and mechanical components found in devices like pacemakers, hearing aids, neurostimulation implants, and insulin pumps. Furthermore, our expertise extends to the inspection of complex, miniaturized, and battery-powered implants, as well as 3D printed items and composite materials, ensuring the highest standards of product integrity.
Ensuring Data Integrity and Regulatory Compliance in Medical Manufacturing
Recognizing the limitations of traditional manual inspection processes, Waygate Technologies provides solutions that facilitate fast and easy electronic record-keeping. Our NDT systems prioritize data integrity and compliance at every stage of information handling – creation, modification, storage, transmission, and retrieval. This focus helps medical device manufacturers meet stringent quality and safety standards, ensuring full traceability and regulatory adherence, ultimately contributing to enhanced product safety and reliability for patients worldwide.
Comprehensive Quality Control Across the Medical Device Lifecycle
Waygate Technologies delivers world-class Computed Tomography (CT) and high-precision dimensional metrology to support full-cycle quality control in the medical technology industry. From detecting flaws and cracks to guaranteeing a perfect fit for every device, our customized inspection solutions address a broad range of applications, including orthopedics, dental implants, and drug delivery scaffolds. By leveraging our 125+ years of industrial inspection experience, we empower OEMs and suppliers to drive process control, ensure product quality and safety, improve product lifespans, and achieve faster product design and increased productivity while lowering costs.
NDT is critical for medical implants because it ensures patient safety by detecting defects before implantation, preventing failures that could lead to severe health issues, costly revisions, and regulatory non-compliance. It guarantees implant integrity, longevity, and adherence to strict quality standards.




